Drug Dispensers
- Published: October 01, 2003, By George Perros, Greystone Assoc.
Until recently, the use of transdermal patches for pharmaceuticals has been limited because only a few drugs have proven effective delivered through the skin — typically cardiac drugs such as nitroglycerin and hormones such as estrogen.
Now, pharmaceutical companies are taking another look at transdermal patches for drug delivery. The availability of improved materials, combined with new patch designs, is creating growth opportunities for companies that manufacture drug patches and their supply chain partners.
How They Work
A skin patch uses a special membrane to control the rate at which the liquid drug contained in the reservoir within the patch can pass through the skin and into the bloodstream. The basic components of any transdermal delivery system include the drug(s) dissolved or dispersed in a reservoir or inert polymer matrix; an outer backing film of paper, plastic, or foil; and a pressure-sensitive adhesive that anchors the patch to the skin. The adhesive is covered by a release liner, which needs to be peeled off before applying the patch to the skin. Drugs administered via skin patches include scopolamine, nicotine, estrogen, nitroglycerin, and lidocaine (see Table I).
Table I
| Drug | Indication or Condition |
|---|---|
| Scopolamine | Motion Sickness |
| Nicotine | Smoking Cessation |
| Estrogen | Menopause, Osteoporosis |
| Nitroglycerin | Angina |
| Lidocaine | Shingles |
| Source: Greystone Assoc. | |

OTC Patch Products
A major market driver for the over-the-counter (OTC) patch is the demographics of the baby boom generation, whose members are entering a phase of their lives in which aches and pains are more common. Their pain may be not only the result of an active life style but also may result from age-related diseases such as arthritis.
These consumers often are well informed, have disposable income, and exhibit a high degree of health consciousness. The aging baby boomers also are more likely than other consumers to have an interest in natural products.
Analgesic and topical patches include the Ace Patch from Becton-Dickenson and the PainPatch from Mentholatum. Both provide topical pain relief for several hours. Many industry analysts believe patches are the fastest growing sub-segment within the OTC category (see Table II).
| Patch | Developer | Description |
|---|---|---|
| Dermaflex | Elan | This is a passive patch design in which the active compound is contained in a hydrogel matrix |
| D-trans | Alza | A passive patch system based on a reservoir architecture |
| E-trans | Alza | A patch design that uses low-power electric current to transport the drug actively through the skin |
| TheraDerm | TheraTech | A reservoir passive patch design |
| TheraPatch | LecTec | A topical non-prescription patch system |
| ThermaCare | Proctor & Gamble | A non-medicated thermal patch for muscle pain management |
| Active Transdermal System | Vyteris | An active patch system using iontophoresis for the delivery of lidocaine and other drugs |
| Source: Greystone Assoc. | ||
Non-medicated patch markets include thermal and cold patches, nutrient patches, skin care patches (a category that consists of two major sub-categories — therapeutic and cosmetic), aroma patches, weight loss patches, and patches that measure sunlight exposure.
The market for thermal patches received significant validation with the entry by Procter & Gamble and the launch of P&G's ThermaCare patch product last year. A company known for its detailed and comprehensive use of consumer market research, P&G's entry and marketing investment into this skin patch segment is sure to capture the attention of other players. We expect to see at least three competing patch products in this sector entering the market in the next twelve months.
>
Patch Design and Technology
There are two major types of transdermal delivery system (TDS) products:
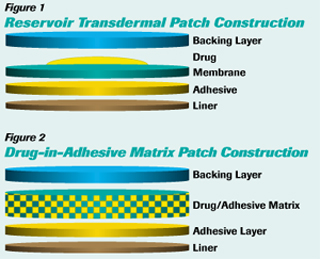
- Reservoir — the active ingredient is held in a solution or suspension between the backing layer and a rate-controlling membrane (see Figure 1).
- Matrix — a solution or suspension dispersed within a polymer or cotton pad in direct contact with the skin and held to the skin by adhesive applied to the perimeter of the system (see Figure 2). Drug-in-adhesive matrix is a refinement in which the polymer (in which the drug is dispersed) is an adhesive.
The container closure system for a TDS is usually a foil pouch made from multilaminates with foil, paper, and heat sealable polyethylene (PE) portions. The foil serves as a vapor barrier, and PE is used for heat sealing purposes.
Emerging and Future Patch Technologies
Extended Wear Patches — Because of the size constraints associated with skin patches, the amount of drug that can be packaged in a single patch is limited, along with the maximum wear time before the patch must be replaced. By developing patch designs that can incorporate higher concentrations of the active drug within the volume constraints of the patch design, while still delivering the drug at the therapeutic rate, an important compliance issue (remembering to change the patch when it becomes exhausted and no longer delivers any therapeutic value) would be removed.
Combination Patches — The concept of combining two or more active ingredients in the same patch has been viewed as a logical progression in patch design since the mid-1990s. The first significant commercial product to capitalize on this concept is the CombiPatch (Noven). It combines estrogen (estradiol) and the progestin (norethindrone acetate) in a twice-weekly transdermal patch. Combination patch designs may include separate reservoirs for each drug or contain multiple drugs in a single reservoir (if there are no chemical and/or biostability issues).
Variable Rate Delivery — Investigations into transdermal patch designs that can vary the rate of drug delivery to respond to physical conditions and physiological changes are underway. Current investigations are focused on bodily changes such as temperature and perspiration rate that may signal the need for higher or lower rates of administration. Polymers in the membrane that respond to temperature changes and allow more or less drug to pass in a given time period are one example.
Transparent Patches — As the use of transdermal patches proliferates, the cosmetic aspects of patch wear have become a potential barrier to growth and thus an issue for transdermal package designers. Transparent patches have come to market that allow the user to wear the patch on an exposed area of skin without the patch being readily noticeable. This feature of transparent patches is particularly attractive in high-temperate climates. The main design feature of transparent patches is the use of a flesh-colored or skin tone backing layer.
Heat-Assisted Patches — Heat is believed to enhance the transdermal delivery of various drugs in a number of ways, including increasing skin permeability and increasing body fluid circulation. Diffusion through the skin is a temperature-dependent process, so raising the skin temperature should increase permeation through the skin.
Future Prospects
Growth in demand for prescription drug patches is being driven by both technology and demographic factors. Improved patch materials and new patch designs are expanding the types and number of therapeutic compounds that can be delivered through the skin.
The aging population in industrialized countries will create a growing market of mature and elderly needing drug therapies and pain management for a variety of maladies associated with the aging process.
Transdermal patches provide an improvement in compliance over oral drugs and injections, since there is no danger of forgetting to take the medication, a common occurrence among elderly patients.
The growing population in developing countries, particularly on the African continent and in parts of the Pacific Rim and South America, will drive demand for patch products in the area of vaccines and contraception.
These factors will contribute to an annual growth rate of 11% for prescription patches over the next five years. Demand for non-medicated patches also will be aided by the population shift to older individuals, as well as by new patch products for treating skin ailments and possessing cosmetic benefits.
George Perros is managing director of Greystone Associates, a medical technology consulting group located in Southern New Hampshire. He earned his undergraduate degree in chemistry, with an emphasis in organic synthesis, and received his MBA from Northeastern Univ. Information contained in this article was excerpted from Greystone's recent market report, “Skin Patches: Prescription, OTC and Non-medicated Markets.” Information on the full report can be obtained by contacting Greystone at 603/673-3444 or by visiting greystoneassociates.org