Trends/Management | Managing Change for Continuous Improvement in a Regulated Industry
- Published: June 07, 2017, By Marcos Batan, Carestream Health Inc.
An ineffective change management process can lead to a chaotic manufacturing environment.
 The need for changes has always been present in business. In most cases, changes are implemented when they are expected to generate a benefit to one or more of the business’s stakeholders.
The need for changes has always been present in business. In most cases, changes are implemented when they are expected to generate a benefit to one or more of the business’s stakeholders.
Those changes can be motivated by a number of different factors. Competition or changes in customer expectations, the business environment, raw materials, suppliers, or within the workforce are just a few examples.
The benefit can be financial (higher profit margins, less operational cash requirements, etc.), to avoid a regulatory intervention (fines, registration suspension, recalls, etc.), make the workplace safer for employees, reduce the company’s environmental footprint, or simply to preserve business reputation. Either if the company changes in a reactive way or in a proactive way, those changes all have one ultimate goal: survival.
The lack of an effective process for managing changes will lead to a chaotic manufacturing environment. Unexplained failures, increased variability, excessive waste, rework, and non-conforming products are common results of that scenario. Those side effects can be summarized by the word: cost.
Several different titles of the Code of Federal Regulations require change management from the companies regulated by them. For those businesses, to have an effective process to manage change is not an option, it is a mandate: 29 CFR 1910.119, 30 CFR 250.1912, 40 CFR 68.75 and 21 CFR 820.30/70 overseen by OSHA, OSMRE, EPA, and FDA respectively are examples of the regulations requiring management of change.
As an example, the FDA executed 2201 inspections in medical device manufacturers (national and international) and issued 3534 form-483 observations in 2013. 113 of them (3.2% of the total) were related to change management.1
In 21 CFD 820.30(i), the Code of Federal Regulations states the following for design changes:
- Each manufacturer shall establish and maintain procedures for the identification, documentation, validation or where appropriate verification, review, and approval of design changes before their implementation.2
In 21 CFD 820.70(b), it states under production and process controls:
- Each manufacturer shall establish and maintain procedures for changes to a specification, method, process, or procedure. Such changes shall be verified or where appropriate validated according to 820.75, before implementation and these activities shall be documented. Changes shall be approved in accordance with 820.40.2
Traditionally, those requirements have been interpreted as a command to control changes or to limit the changes to the absolutely necessary. The necessary changes were often only the ones imposed by external factors like obsolete pieces of equipment and changes to raw materials implemented by vendors. Therefore, the change management system was essentially reactive.
That interpretation, associated with the registration requirements, drove to what the FDA classified as “static manufacturing.” In its report, “Innovation and Continuous Improvement in Pharmaceutical Manufacturing; Pharmaceutical CGMP for the 21st Century,” the FDA evaluated the mindset in the pharmaceutical industry as follows:
- Static manufacturing can create, or is a result of, a mind-set that “the product is approved and validated – do not change.”3
In that environment, change control processes were implemented with a heavy focus on complex documentation and compliance, what was a significant disincentive to change. The philosophy was to reduce the regulatory risk by limiting change.
The change control approach had undesirable consequences as well. The heavy focus on limiting change and the heavy weight placed on compliance and on documentation significantly hindered innovation and continuous improvement. Low productivity levels, high percentages of non-conforming product, high waste, rework, and inventory levels followed.
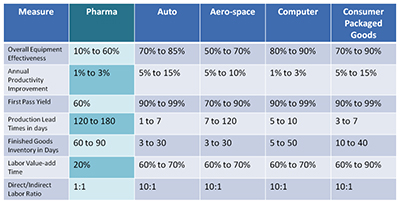
The table above shows estimates from McKinsey Operations Practice, published by Shah in 20114. It shows that the pharmaceutical industry lags similar industries in key measures of operating performance.
While several factors might have influenced that performance, such as heavier focus in R&D in detriment to manufacturing, it is reasonable to assume that lack of innovation and continuous improvement had a determining impact over those operational results.
In the first decade of the 21st century, the FDA completely transitioned the terminology used on its guidance for the industry documents from change control to change management. “The Q10 guidance document (FDA/ICH Guidance for the Industry, Q10 Pharmaceutical Quality System -7) demonstrated a complete transition from attempting to control change to managing changes for process improvement. The goals allow pharmaceutical manufacturers, not only to achieve compliance but to gain business advantage.”5

In essence, the FDA recognized that the health of the US citizens depends on the availability of not only safe and effective but also affordable medicines. Continuous improvement and innovation are key to that goal. It also realized that without flexibility and change, there is no improvement.
With change management, the focus shifted from restriction of change to oversight of the change process supported by sound risk assessment and testing plans enabled by good process understanding.
While change control had an unbalanced focus on compliance and documentation, change management rebalanced the focus toward people and the assessment of the change.
The sections below are not intended to be a comprehensive list of what needs to be done to implement a change management system. Instead, the sections offer the operation’s perspective of how a compliant system can be implemented while still fostering a culture of continuous improvement.
Documentation
There is a common saying in the regulated industry: if you can’t prove it, it never happened. Therefore, the documentation requirements should reflect the minimum information necessary to satisfy the regulatory mandates.
A very common trend is to make forms extensive and complex under the false premise of making them more effective and therefore compliant. In reality, complex documents only increase the regulatory risk as it is more difficult for change leaders to fill them consistently and in alignment with the company’s own procedures.
Complex documents also drive undocumented changes or represent a disincentive to change. The first is another regulatory risk while the latter is a barrier to improvement.
For the documentation not to represent a threat to the culture of continuous improvement, it needs to be perceived as a useful tool that will help the project leaders plan and execute their changes.
Change Assessment
Based on process knowledge, a thorough change assessment is a critical step of a successful change management process.
It should start by listing all the expected benefits and how they can be measured. A detailed risk assessment should follow to determine what risks to assess.
It is also very important that the test plan be robust enough to detect unexpected side effects of the change. Those effects can be positive (and should be added to the list of benefits of the change) or negative. In both cases, they will help determine the true net benefit of the change.
It is often difficult to fully predict and assess the risks associated with changes, so a short test plan may not catch the impact of all factors. This includes issues that take place over time as parts wear, equipment gets dirty, or variability within specification presents itself.
People
People are perhaps the most neglected component of any system. As the real drivers of the process, people should receive significant attention.
All employees expected to participate in the change management process should understand the rationale behind the change management process and the regulation-driven requirements. In that way, the fear of the unknown can be eliminated, helping strengthen the continuous improvement culture.
A significant effort should be made toward making the change management process as easy to follow as possible. Simplification of forms and the automation of repetitive tasks are examples of how the system can be made easier to follow.
An employee’s time is much better spent planning, assessing, and executing the change instead of waiting for long chains of approval. Therefore, any effort should be made toward reducing the approval lead times.
Most importantly, employees should be constantly stimulated to actively and proactively look for changes that will deliver the benefits the company is looking for.
The Leadership Role
The leadership position in relationship to change will determine the success or the failure of driving a continuous improvement culture. Therefore, leaders should be supportive of continuous improvement and innovation.
Since people are naturally resistant to change, it is important for leaders to share the expected benefits of changes. Sharing such information will make people more likely to embrace changes that have a positive impact in the company.
Conclusions
An ineffective change management process can lead to a chaotic manufacturing environment and the side effects can be summarized by an increased operational cost.
A number of different titles of the Code of Federal Regulations require change management. As a consequence, businesses regulated by those have to have a robust change management process.
Traditionally, those requirements have been interpreted as a command to control changes or to limit the changes to the absolutely necessary. That interpretation also led to additional operational costs as it hindered innovation and continuous improvement.
Even some regulatory agencies (like the FDA) started to understand the importance of continuous improvement and innovation. As a result, the focus shifted from change control to a fully functional change management system.
The implementation of a change management process requires a better balance between compliance, documentation, assessment and people.
References
1. 2013 Annual FDA Medical Device Quality System Data. FDA Form 483 Observations and Warning Letter Citations. Retrieved from: http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-afda-orgs/documents/document/ucm416501.pdf
2. CFR – Code of Federal Regulations Title 21. Updated in April 1, 2015.
3. Innovation and Continuous Improvement in Pharmaceutical Manufacturing. Pharmaceutical CGMPs for the 21st Century. Retrieved from: http://www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4080b1_01_manufSciWP.pdf
4. Shah, Vibhakar (2011). PAT, QbD and Continuous Process Verification – The 21st Century Opportunity. Retrieved from https://www.gmp-navigator.com/guidemgr/files/2011__PAT_V_Shah_FDA.pdf.
5. Bhandola, P. (2015) Leveraging Change for Continuous Process Improvement. Pharmaceutical Technology, volume 38, issue 10.
about the author
Marcos Batan has 20 years of experience in the manufacturing of medical devices; 13 of which were spent in managerial positions that covered most operational and technical aspects of manufacturing. His technical experience includes aqueous coating and manufacturing of oriented films. He is currently the technical manager of Carestream’s Polyester Mfg. Dept. in Windsor, CO. Marcos holds a Chemical Engineering degree from Universidade de Sao Paulo (Brazil) and a graduate degree in Business Administration from Fundacao Getulio Vargas (Brazil). Contact him at This email address is being protected from spambots. You need JavaScript enabled to view it..
This article is adapted from the author’s presentation at the AIMCAL 2016 Web Coating and Handling Conference, Memphis, TN.